Overview
This article addresses the body of research concerning subtypes of autism spectrum disorder including pervasive developmental disorders. Although positron emission tomography (PET) scanning has contributed to the knowledge about autism spectrum disorder [1, 2] (see the image below), the procedures described in this article are research protocols. Currently, PET scanning and other nuclear medical procedures are not indicated in the evaluation, diagnosis, and treatment of individuals who may have autism spectrum disorder. The clinical role of quantitative EEG and brainstem auditory-evoked potential testing also is undetermined and requires further evidence.
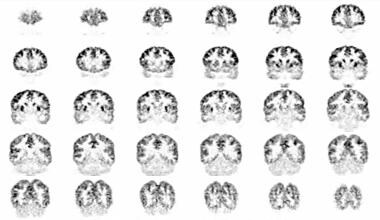 This image was obtained approximately 30 minutes after intravenous administration of 4.15 mCi of [18F]fluoro-2-deoxyglucose (FDG) to a 10-year-old boy with autism spectrum disorder and unspecified intellectual disabitliy using auto-attenuation with a 24-minute acquisition period. Coronal sections are shown from anterior to posterior; the left side of the figure corresponds to the right side of the patient. Courtesy of Rashid A Fawwaz, MD, PhD, The Kreitchman PET Center, Columbia-Presbyterian Medical Center, New York, NY.
This image was obtained approximately 30 minutes after intravenous administration of 4.15 mCi of [18F]fluoro-2-deoxyglucose (FDG) to a 10-year-old boy with autism spectrum disorder and unspecified intellectual disabitliy using auto-attenuation with a 24-minute acquisition period. Coronal sections are shown from anterior to posterior; the left side of the figure corresponds to the right side of the patient. Courtesy of Rashid A Fawwaz, MD, PhD, The Kreitchman PET Center, Columbia-Presbyterian Medical Center, New York, NY.
Autism and autism spectrum disorder
Autism spectrum disorder [3] is a class of developmental disorders that presents in early childhood and is characterized by marked abnormalities in language, communication, and social interactions and by a restricted and peculiar range of interests and activities. Thus, obsessions and compulsions [4] characterize the behaviors of many people with autism. For 2016, the prevalence of autism spectrum disorder among children 8 years of age was 18.5 per 1,000 (one in 54) in the United States. Males are 4.3 times more likely than females to be identified with autism. [5, 6, 7, 8, 10] Most people with autism also exhibit intellectual disabilities, and approximately one third of people have seizure disorders. Asperger disorder [11] refers to the presence of the social behavior and repetitive activities of autism spectrum disorder without intellectual disability (ie, high-functioning autism).
Complications in the prenatal, perinatal, and postnatal periods have been described in some cases of autism spectrum disorder. [12, 13, 14] Exposure to toxins, poisons, residues, and other contaminants is one of the mechanisms hypothesized to cause autism and related conditions. Although evidence exists that toxic exposure may play a role in isolated cases of autism spectrum disorder, no convincing evidence that toxic exposure is instrumental in the causation of autism spectrum disorder in the population in general has been reported. In particular, anecdotal reports that autism spectrum disorder developed in children who received immunization to measles, mumps, and rubella have not been confirmed in the general population. Thus, immunization is not associated with the development of autism spectrum disorder in general. For this reason, routine immunizations, including immunization for measles, mumps, and rubella, are recommended for the general population.
Autism spectrum disorder includes idiopathic and genetic subtypes. Autism spectrum disorder includes idiopathic conditions formerly classified as autism, [3] autistic disorder [3] , and Asperger disorder, childhood disintegrative disorder (Heller syndrome), and other pervasive developmental disorders. Autism spectrum disorder also includes genetic disorders such as fragile X syndrome, Rett syndrome, and tuberous sclerosis. Thus, autism spectrum disorder includes conditions with some features of autism and without all criteria required for autism spectrum disorder. Additionally, some people with autism spectrum disorder exhibit obsessions, [4] compulsions, [4] and catatonia. [15, 16, 17] Autism spectrum disorder is probably a heterogeneous condition with multiple causes, some of which are unknown, i.e., idiopathic.
Since autism spectrum disorder exhibits a drastic increase in incidence and early intervention is key to optimal outcome, referral of children for specialized evaluation and treatment is recommended by the American Academy of Pediatrics as soon as symptoms or signs are observed. [88, 89]
Diagnostic criteria
A major concern in the interpretation of reports about PET scans in autism spectrum disorders is the accuracy and the precision of the diagnoses. The widely used systems of nomenclature, including the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) [18] ; the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) [19] ; the International Classification of Diseases, Ninth Revision, Clinical Modification, Fourth Edition (ICD-9-CM) [20] ; the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) [21] and the International Classification of Diseases, 11th Revision (ICD-11) [90] list different criteria for autism spectrum disorder.
These criteria are listed in an outline fashion that challenges inexperienced readers and are prone to misinterpretation. Therefore, attempts by inexperienced individuals to diagnose autism spectrum disorder by the application of criteria from these manuals are fraught with error. The diagnostic criteria for autism spectrum disorder are currently in flux. The lack of uniformity in diagnostic criteria and classification systems leads to confusion in the interpretation of studies using the common nomenclatures [21, 19, 18, 20, 90] and other varied schemes.
The changes in the diagnostic criteria for autism spectrum disorder and related conditions probably contribute to the apparent increase in the prevalence of these disorders. [22] Nevertheless, the reported increments in the incidence of autism spectrum disorder provide evidence that autism spectrum disorder constitutes a public health threat of major proportions. Likely, revision of this contribution will soon be needed when a set nomenclature is in place.
Specialized diagnostic procedures have been developed for autism spectrum disorder. The diagnostic tools for autism spectrum disorder require training in order to attain a reliable administration. The detailed description of the procedures to diagnose autism spectrum disorder is beyond the scope of this article. When in doubt about the possible presence of these disorders, please refer the individual to a clinician experienced in the diagnosis of autism spectrum disorder for a definitive evaluation.
Evaluation of Autism Spectrum Disorder
Clinical symptoms of autism spectrum disorder include problems with social interaction, problems in making friends, and limited nonverbal communication. Because social deficits are a hallmark of autism spectrum disorder, researchers are investigating the nature of nonverbal communications, including facial recognition and interpretation of affect exhibited by facial expressions. The understanding of hand gestures and eye gaze provides crucial clues about the feelings and intentions of others. Persons with autism spectrum disorder exhibit deficits in facial perception. Instead of identifying people on the basis of overall facial configuration, persons with autism spectrum disorder use the lower face, the mouth, and other specific portions of the face to identify others. Thus, people with autism spectrum disorder actually may identify the faces of people by focusing on the objects that form the face rather than the whole person.
Since autism spectrum disorder exhibits a drastic increase in incidence and early intervention is key to optimal outcome, referral of children for specialized evaluation and treatment is recommended by the American Academy of Pediatrics as soon as symptoms or signs are observed. [88, 89]
Brain and neurologic anomalies
Megalocephaly has been reported in a few cases of autism spectrum disorder. Thus, a subset of individuals with autism spectrum disorder may be characterized by this finding.
The cerebella of some individuals with autism spectrum disorder demonstrate hypoplasia, [23, 24] whereas others demonstrate hyperplasia. Additionally, oculomotor studies have provided evidence of neocortical dysfunction of the prefrontal cortex and connections to the parietal lobe. Other subgroups of autism spectrum disorder exhibit neocortical dysfunction and cerebellar dysfunction.
Perception is accomplished in the brain by means of a parietal pathway for spatial and motor function and a temporal pathway for identification of objects, faces, and gestures. Anomalies in the amygdala and other structures of the medial temporal lobe have been demonstrated repeatedly in autism spectrum disorder, suggesting involvement of the temporal pathway for visuoperceptual processing.
Functional magnetic resonance imaging (fMRI) of people with autism spectrum disorder demonstrates reduced activation in the fusiform gyrus, the portion of the brain associated with facial recognition, and increased activation of adjacent portions of the brain associated with recognition of objects. The enlarged right superior temporal gyri observed in a cohort of males with high-functioning autism spectrum disorder provides a possible anatomic basis for the visual and social deficits of those individuals. [25] Functional magnetic resonance imaging (fMRI) also suggests that in some individuals with Asperger syndrome (high-functioning autism spectrum disorder), dysfunctional connections among limbic and paralimbic regions, the cerebellum, and the extrastriate visual cortices occur during the process of identification of the emotion expressed by faces and the sex of the face.
Rett syndrome is a subtype of autism spectrum disorder associated with a specific genetic mutation presenting in early childhood primarily in girls. Children with Rett syndrome exhibit normal development in the first year of life. They typically fail to attain milestones between ages 1 and 6 years. They exhibit profound intellectual disability and typically require assistance in the activities of daily living. Almost all individuals with Rett syndrome display abnormalities of the methyl-CpG-binding protein 2 (MeCP2) gene. [26] Women with Rett syndrome demonstrate less acetylcholine in the brain than healthy women. [27] Reductions in acetylcholine are correlated with reduced abilities to perform activities of daily living in women with Rett syndrome. [27]
See the image below.
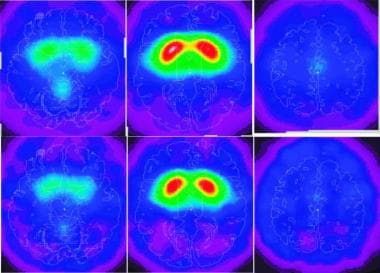 Visual expressions of mean uptake on single-photon emission-computed tomography (SPECT) 24 h following the intravenous administration of approximately 333 MBq (9 mCi) (2)-5-[123I]iodobenzovesamicol ([123I]IBVM), a radioligand for vesicular acetylcholine transporters to eight healthy adults (upper row) and four women with Rett syndrome (lower row). The left of each panel corresponds to the left of the brain. The panels represent transverse sections of the brain at the level of the cerebellum (left), striatum (center), and cingulated gyrus (right). The lower row illustrates the reduced uptake in the women with Rett syndrome in the vermis and the bilateral precentral cortices (lower left panel), the striatum (lower central panel), and the middle cingulate gyrus (right lower panel). Used with permission from Brasic JR, et al. Correlation of the vesicular acetylcholine transporter densities in the striata to the clinical abilities of women with Rett syndrome. Synapse. Jun 2012;66(6):471-82.
Visual expressions of mean uptake on single-photon emission-computed tomography (SPECT) 24 h following the intravenous administration of approximately 333 MBq (9 mCi) (2)-5-[123I]iodobenzovesamicol ([123I]IBVM), a radioligand for vesicular acetylcholine transporters to eight healthy adults (upper row) and four women with Rett syndrome (lower row). The left of each panel corresponds to the left of the brain. The panels represent transverse sections of the brain at the level of the cerebellum (left), striatum (center), and cingulated gyrus (right). The lower row illustrates the reduced uptake in the women with Rett syndrome in the vermis and the bilateral precentral cortices (lower left panel), the striatum (lower central panel), and the middle cingulate gyrus (right lower panel). Used with permission from Brasic JR, et al. Correlation of the vesicular acetylcholine transporter densities in the striata to the clinical abilities of women with Rett syndrome. Synapse. Jun 2012;66(6):471-82.
Role of serotonin in development of affiliation and cognition
Multiple aspects of growth, including affiliation and cognition, are hypothesized to depend on serotonin during development. Affiliate behaviors in animals, including humans, appear to depend on serotonin, the interaction of serotonin and opioid peptides, and the neurohypophyseal hormones oxytocin and vasopressin. Several lines of evidence in animals and humans suggest that serotonin is crucial to development and maintenance of normal affiliate behaviors. Additional evidence suggests that serotonin regulates development and function of the brain and that disrupted development of the serotonin system leads to autism and intellectual disability. Adequate serotonin appears crucial early in development.
Failure to experience normal social interactions in infancy and childhood may prevent development of normal socialization at later stages of life, as in rats. Thus, during a critical period in gestation, infancy, and early childhood, the presence and effects of serotonin may be crucial for development of social skills in humans. Inadequate stimulation of serotonin to portions of the human brain early in life may create abnormalities of regulation of serotonin metabolism that cannot be corrected later. Thus, dysregulation of serotonin metabolism may be permanent in the brains of people deprived of necessary serotonin influences at key stages of early development. Furthermore, facilitation of serotonin appears to promote social confidence and the ability to relate to others. Therefore, persuasive evidence indicates that serotonin exerts a key role early in life on portions of the brain necessary for development of affiliation and cognition.
A strong genetic etiology probably exists for many cases of autism. Genetic studies provide evidence for the association between serotonin transporter and autistic disorder. A study of a German sample of people with autism spectrum disorder revealed higher frequency and preferential transmission of the long allele of a common polymorphism, the serotonin transmitter gene-linked polymorphism region, in the upstream regulatory region of the serotonin transporter. However, another study with an international sample of subjects demonstrated no association of the serotonin transporter gene and autism spectrum disorder. Another study did not confirm the association of the serotonin type 7 receptor with autism spectrum disorder. Ethnic differences in study populations may account for some of the discrepancies between study results.
Past medical and social information
A variety of personal and clinical data is relevant to the analysis of studies of individuals with autism spectrum disorder. Specifically the presence of claustrophobia or scoliosis may interfere with the ability to hold still for scans. Additionally the use of nicotine, caffeine, alcohol, and drugs may have short-term and long-term effects on the brain such that current and past use of substances is relevant. The presence of other medical, neurologic, psychiatric, and psychologic conditions may affect the scans of the individual. Therefore, knowledge of all current and previous physical and emotional disorders is crucial.
The history of a suicide attempt is important because subjects experience both physical and psychologic stress before, during, and after imaging scans. Individuals who are acutely suicidal merit inpatient psychiatric hospitalization. Generally, subjects who are suicidal are not appropriate for scans for research purposes. The special needs of research on suicide are beyond the scope of this article.
Screening for PET Scanning
In order to obtain relevant screening information, patients who may undergo PET scanning complete a questionnaire for subjects (see the image below for a printable version) to be reviewed by the clinician and the technologist before commencing PET scans. This form can be sent to individuals as part of the screening process. Thus, pertinent information necessary to determine the appropriate PET scan for the patient is readily available for the clinician to streamline the medical history and physical examination.
Questionnaire for Subjects Form
Patients who would not benefit from PET scans can be excluded from further participation if the presence of exclusionary criteria is identified on review of the Questionnaire for Subjects form. For clinical studies, the presence of positive findings on this questionnaire indicates the need for tailoring the future studies to the needs and limitations of the individual.
After review of the Questionnaire for Subjects Form confirms that the person is suitable for further evaluation, then a medical history and physical examination must be accomplished before nuclear studies are performed. Additionally, rigorous recording of demographic data, [28] including ethnicity, [29] is crucial to the accurate characterization of clinical patients and research subjects for clinical, research, administrative, and educational purposes.
Documentation of medical history and physical examination in preparation for PET scans
Systemic recording of the key components of the medical history of the patient, using a Nuclear Medical History Recording Form (see the first image below for a printable version) and examinations, using the Nuclear Medical Physical Examination Recording Form (see the second image below for a printable version) are helpful to verify that an adequate assessment has been obtained for nuclear medical procedures. This information can readily be entered into the permanent medical record of the patient. The forms can be scanned to be transmitted and archived as permanent medical records.
Nuclear Medical History Recording Form
Nuclear Medical Physical Examination Recording Form
Lateral preferences evaluation
Typically, patients with autism spectrum disorder and related conditions have few findings on medical history and physical examination. The presence of positive findings on medical history and physical examination indicates the need to verify the absence of exclusionary criteria for PET scans. Also, the presence of positive findings on medical history and physical examination indicates the need for consideration of possible contraindications for clinical studies. The needs and limitations of each individual must be considered before proceeding with nuclear studies.
Because the findings in the brains of people with autism spectrum disorder and related conditions may be lateralized, identification of the lateral preferences of the subject is a crucial component of the examination of each subject. Denckla developed a quick procedure, suitable for clinical settings, that is used to identify the lateral preferences for the use of the eye, the hand, and the foot in children, adolescents, and adults. [30] Before scans of the brain are performed, the Lateral Preferences Examination Form (see image below for a printable version) is completed for all subjects to identify the preferred side for the eye, the foot, and the hand.
Lateral Preferences Examination Form
The examiner scores the Lateral Preferences Examination in vivo. In addition to the live rating by the examiner, the Lateral Preferences Examination is videotaped for later blind rating by experts unfamiliar with the status of the subject. The blind videotape rating of the Lateral Preferences Examination is useful to determine reliability of the live ratings of the examination.
Additionally, blind rating of the videotape of the Lateral Preferences Examination is useful to protect against any left-right dissociation in the examiner. Examiners may experience confusion themselves in determining the side used by the subjects for each task. Therefore, rating of videotaped assessments is a crucial component of research studies of scans of the brain.
PET Scanning Overview
Positron emission tomography (PET) scanning is a functional imaging technique that visualizes physiologic structures in relation to events, such as performance of activities and administration of chemical agents. [31, 32, 33] PET scanning is used as a tool to visualize the physiological functions of the brain after administration of radioligands.
 This figure shows a woman positioned to enter the chamber of a high-resolution tomography (HRRT). (Alveena B. Syed photographed by James Robert Brašić)
This figure shows a woman positioned to enter the chamber of a high-resolution tomography (HRRT). (Alveena B. Syed photographed by James Robert Brašić)
PET scanning also permits mapping of receptors throughout the body. This is particularly important with regard to autism spectrum disorder, because PET scanning can identify receptors for specific neurotransmitters in vivo. Some groups of individuals with autism spectrum disorder have dysfunction of serotonin, dopamine, nicotine, and other neurotransmitters in portions of the central nervous system (CNS). PET can assess the function of receptors for serotonin and other neurotransmitters throughout the brain. These findings are important, because they provide clues to novel therapeutic interventions.
PET scanning is also used in the study of other diseases, such as identifying malignant growths and other disorders in the brain, other portions of the CNS, and other parts of the body. [34, 35, 36] Because this imaging modality can measure regional cerebral glucose metabolism, PET scanning with [18F]fluoro-2-deoxyglucose (FDG) can be used to demonstrate interictal temporal hypometabolism consistent with marked improvement after surgical treatment in patients with complex partial seizures.
PET Scanning and Metabolism in Autism Spectrum Disorder
Positron emission tomography (PET) scanning has demonstrated multiple deficits in individuals with autism spectrum disorder; however, no single finding has characterized all people with autism spectrum disorder. The results have varied from individual to individual. Autism spectrum disorder is characterized by a syndrome, specifically a constellation of symptoms (patient complaints), signs (objective findings of the examiner), laboratory findings, family history, and a particular course. [37, 38] There are likely multiple etiologies for autism. Autism spectrum disorder represents a heterogeneous group of subtypes with varying biological causes.
When PET scanning is used with [18 F]fluoro-2-deoxyglucose (FDG), the anterior rectal gyrus has been found to be larger on the left side than the right side in some people with autism spectrum disorder, a finding opposite to the asymmetry seen in healthy individuals. Some people with autism spectrum disorder also exhibit increased glucose metabolic rate in the right posterior calcarine cortex and decreased glucose metabolic rate in the medial frontal region, left posterior putamen, and left medial thalamus. Simultaneous correlation of the structures on PET scanning and magnetic resonance imaging (MRI) has established that the right anterior cingulate gyrus is smaller and metabolically less active in a small group of high-functioning adults with autism spectrum disorder.
For example, the images that follow were obtained approximately 30 minutes after intravenous (IV) administration of 4.15 mCi of FDG to a 10-year-old boy with autism spectrum disorder and unspecified intellectual disability using autoattenuation with a 24-minute acquisition period.
The image below demonstrates coronal sections from anterior to posterior; the left side of the figure corresponds to the right side of the patient.
 This image was obtained approximately 30 minutes after intravenous administration of 4.15 mCi of [18F]fluoro-2-deoxyglucose (FDG) to a 10-year-old boy with autism spectrum disorder and unspecified intellectual disabitliy using auto-attenuation with a 24-minute acquisition period. Coronal sections are shown from anterior to posterior; the left side of the figure corresponds to the right side of the patient. Courtesy of Rashid A Fawwaz, MD, PhD, The Kreitchman PET Center, Columbia-Presbyterian Medical Center, New York, NY.
This image was obtained approximately 30 minutes after intravenous administration of 4.15 mCi of [18F]fluoro-2-deoxyglucose (FDG) to a 10-year-old boy with autism spectrum disorder and unspecified intellectual disabitliy using auto-attenuation with a 24-minute acquisition period. Coronal sections are shown from anterior to posterior; the left side of the figure corresponds to the right side of the patient. Courtesy of Rashid A Fawwaz, MD, PhD, The Kreitchman PET Center, Columbia-Presbyterian Medical Center, New York, NY.
The image below demonstrates axial sections from superior to inferior; the left side of the figure corresponds to the right side of the patient.
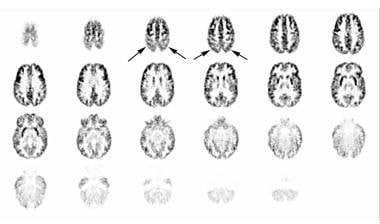 This image was obtained approximately 30 minutes after intravenous administration of 4.15 mCi of [18F]fluoro-2-deoxyglucose (FDG) to a 10-year-old boy with autism spectrum disorder and unspecified intellectual disability using auto-attenuation with a 24-minute acquisition period. Axial sections are shown from superior to inferior; the left side of the figure corresponds to the right side of the patient. Hypometabolism, greater on the left than the right, is demonstrated in the temporal and parietal regions. This is particularly prominent in the third and fourth images. Courtesy of Rashid A Fawwaz, MD, PhD, The Kreitchman PET Center, Columbia-Presbyterian Medical Center, New York, NY.
This image was obtained approximately 30 minutes after intravenous administration of 4.15 mCi of [18F]fluoro-2-deoxyglucose (FDG) to a 10-year-old boy with autism spectrum disorder and unspecified intellectual disability using auto-attenuation with a 24-minute acquisition period. Axial sections are shown from superior to inferior; the left side of the figure corresponds to the right side of the patient. Hypometabolism, greater on the left than the right, is demonstrated in the temporal and parietal regions. This is particularly prominent in the third and fourth images. Courtesy of Rashid A Fawwaz, MD, PhD, The Kreitchman PET Center, Columbia-Presbyterian Medical Center, New York, NY.
The following image demonstrates sagittal images of the patient from right to left. Hypometabolism, greater on the left than the right, is demonstrated in the temporal and parietal regions. This is particularly prominent in the third and fourth depictions in the previous image, above.
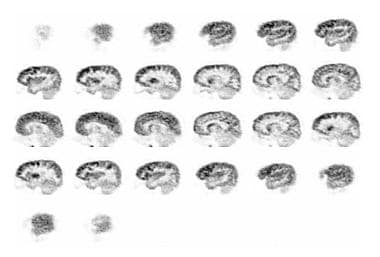 This image was obtained approximately 30 minutes after intravenous administration of 4.15 mCi of [18F]fluoro-2-deoxyglucose (FDG) to a 10-year-old boy with autism spectrum disorder and unspecified intellectual disability using auto-attenuation with a 24-minute acquisition period. Sagittal images are seen from right to left. Courtesy of Rashid A Fawwaz, MD, PhD, The Kreitchman PET Center, Columbia-Presbyterian Medical Center, New York, NY.
This image was obtained approximately 30 minutes after intravenous administration of 4.15 mCi of [18F]fluoro-2-deoxyglucose (FDG) to a 10-year-old boy with autism spectrum disorder and unspecified intellectual disability using auto-attenuation with a 24-minute acquisition period. Sagittal images are seen from right to left. Courtesy of Rashid A Fawwaz, MD, PhD, The Kreitchman PET Center, Columbia-Presbyterian Medical Center, New York, NY.
The above findings suggest functional bases for the abnormalities in assessing environmental visual and emotional cues in individuals with autism spectrum disorder. Reversal in the size of the anterior rectal gyrus and reversal of hemispheric dominance for language in some people with autism spectrum disorder suggest possible bases for the social, emotional, and language deficits.
In people with tuberous sclerosis and autistic disorder, scans with [18F]FDG demonstrate that glucose metabolism is decreased bilaterally in the lateral temporal gyri and increased bilaterally in deep cerebellar nuclei. [39, 40, 41]
[18F]FDG-PET, autism spectrum disorder, and facial port-wine stains
Chugani and colleagues reported that [18F]FDG-PET characterizes a group of children with evidence of autism spectrum disorder and unilateral facial port-wine stains. [42] [18F]FDG-PET scans differentiate children with autism spectrum disorder and facial port-wine stains from children with autism spectrum disoder without facial port-wine stains. In children with autism spectrum disorder and facial port-wine stains, decreased metabolism on FDG-PET was observed bilaterally in the medial temporal regions, the frontal cortices, the anterior cingulate gyri, the cerebellum, and the right temporal cortex. [42, 8]
In contrast, children with autism spectrum disorder without facial port-wine stains showed decrements in metabolism on [18F]FDG-PET that were mild in the left medial temporal regions and more pronounced in the right temporal cortices. [42] Furthermore, children with autism spectrum disorder without facial port-wine stains showed reversals of the normal frontal temporal asymmetry in metabolism on [18F]FDG-PET. [8] These observations of a small sample of patients await confirmation by other groups. [42]
PET, autism, and infantile spasms
Among a group of children with infantile spasms, those who develop autism spectrum disorder exhibited reduced metabolism in the frontal, parietal, and temporal lobes. [43] These results suggest that reduced frontal, parietal, and temporal metabolism may predispose children with infantile spasms to develop autism spectrum disorder. [43]
PET Scanning of the Serotonergic System
The following will be discussed in this section:
-
Serotonin metabolism in autism spectrum disorder
-
Serotonin dysfunction in comorbid disorders
-
Peripheral serotonin abnormalities in autism spectrum disorder
-
PET scan studies of the serotonergic system in autism spectrum disorder
-
Treatment studies with serotonergic agents
Serotonin metabolism in autism spectrum disorder
The role of serotonin in development of affiliation and cognition and genetic evidence for serotonin anomalies in autism spectrum disorder were discussed in Evaluation of Autism Spectrum Disorder.
Abnormalities of serotonin metabolism play an important role in individuals with autism spectrum disorder. Whole-blood serotonin is often elevated in children with autism spectrum disorder and normal intelligence. Also, in boys with autism spectrum disorder, serotonin synthesis typically is decreased in the frontal region and thalamus on one side of the brain and increased in the dentate nucleus of the opposite cerebellum. Therefore, serotonin synthesis appears to vary distinctly in subgroups of people with autism spectrum disorder that are based on sex and intelligence.
Elucidation of the role of serotonin in development and maintenance of the symptoms and signs of autism spectrum disorder will facilitate development of treatments targeted to correct the specific deficits of each subtype, including gene transfer strategies. The fact that one third of people with autism spectrum disorder demonstrate elevated blood serotonin levels gives reason to suspect that some people with autism spectrum disorder may have abnormalities in the serotonergic pathways in the brain and other parts of the body. Chugani reviewed the evidence for anomalous development of the serotonin system in the brain in autism spectrum disorder. [44, 45]
Abnormalities in the metabolism of serotonin in the brain characterize a large group of individuals with autism spectrum disorder. The value of clarification of the nature of the serotonergic dysfunction has been recognized; however, most previous studies indirectly used peripheral serotonin measures (eg, blood, urine) that are not associated consistently with the diagnosis of autism spectrum disorder.
Further evidence suggests that serotonin receptors and transporters do not function properly in individuals with autism spectrum disorder. Elucidation of anomalies in the functioning of serotonin transporters and receptors in autism spectrum disorder will form the basis of specific therapy targeted to meet identified deficits. Dysfunctions of serotonin receptors and transporters characterize distinct biologic classes of individuals with autism spectrum disorder.
In recent years, positron emission tomography (PET) scanning has been employed to suggest the function of portions of the brain associated with the deficits observed through neurophysiologic and neuropsychologic examination. However, quantitative interpretation of this method has not been confirmed.
Alpha-[11C]methyl-L-tryptophan ([11C]AMT), a tracer for measuring the synthesis of serotonin, has been used with PET scanning. [46, 47, 48, 49, 50, 68, 69, 70] Studies using PET after administration of [11C]AMT, primarily of children with autism spectrum disorder, have demonstrated striking anomalies of serotonin synthesis in boys. Children with autism spectrum disorder demonstrate several patterns of abnormal cortical development, including the left cortex and the right cortex. Left cortical [11C]AMT decreases in children with autism spectrum disorder are associated with marked language disorders. [50, 45]
Additional evidence pointing to dysregulation of serotonin metabolism in autism spectrum disorder includes the likely pathophysiology of the sleep abnormalities in people with autism spectrum disorder.
Serotonin dysfunction in comorbid disorders
Evidence to support the hypothesis that serotonin dysfunction causes some cases of autism spectrum disorder comes from studies of the neurophysiology of other disorders commonly encountered in people with autism spectrum disorder, including mood disorders, Tourette disorder, and obsessive-compulsive disorder. [4] Dysregulation of serotonin metabolism is implicated in the pathogenesis of major depression and obsessive-compulsive disorder. The rates of depression, obsessions, [4] and compulsions [4] often are increased in populations of individuals with autism spectrum disorder.
Some individuals with Tourette disorder have evidence of dysfunction of transmission of dopamine [92] and serotonin. [91] Dysregulation of serotonin in the subthalamus has been suggested in some studies of Tourette disorder. Interaction of abnormal functions of the dopaminergic and serotonergic systems may contribute to Tourette disorder.
Peripheral serotonin abnormalities in autistic disorder
Elevated levels of whole-blood serotonin have consistently characterized a group of approximately one third to two thirds of individuals with autism spectrum disorder and their parents and siblings, despite ethnic variations. However, some samples of children with autism spectrum disorder have demonstrated decreased serotonin uptake. The presence of a familial deficit in serotonin metabolism is suggested further by the finding that parents of children with autism spectrum disorder who themselves (the parents) have elevated whole-blood serotonin levels also have more depression, obsessions, and compulsions. [51]
The demonstration of hyperserotonemia in children with autism spectrum disorder without intellectual disability and hyposerotonemia in children with intellectual disability without autism spectrum disorder raises the possibility that peripheral serotonin levels in autism may vary according to levels of intelligence of the subjects. [52] This finding suggests that children with autism spectrum disorder with intellectual disability may be biologically distinct, in terms of dysfunction of the serotonin system, from children with autism spectrum disorder without intellectual disability.
Reduction of serum tryptophan to large neutral amino acid ratios characterizes a group of children with autism spectrum disorder, [53, 54] suggesting decreased availability of brain tryptophan, which is a precursor of serotonin. A tripeptide that stimulates uptake of serotonin into platelets has been demonstrated in the urine of almost two thirds of a sample of children with autism spectrum disorder.
Decreased central serotonin binding due to antibodies to central serotonin (5-HT1A) receptors and decreased central serotonergic response have been proposed as mechanisms that mediate hyperserotonemia in some individuals with autism spectrum disorder. However, autoantibodies against 5-HT1A receptors were not demonstrated in a small group of children with autism disoder. [55]
General serotonin receptor binding, without determination of serotonin receptor subtype, is inhibited by the plasma of children with autism spectrum disorder without intellectual disability but is not inhibited by the plasma of children with intellectual disability without autism spectrum disorder or by the plasma of children with typical development. These findings are apparently due to the presence of blocking antibodies and not to high plasma concentrations of serotonin. Therefore, serotonin may trigger a B-cell–mediated autoantibody response to the serotonin receptor in children with autism spectrum disorder.
The contribution of antiserotonin receptor antibodies to the symptomatology of autism spectrum disorder is suggested by the motor inhibition seen in mice treated with antiserotonin antibodies. [56] Although several studies have used the resemblance of serotonin type 2A receptors in human platelets and in the human frontal cortex to indirectly infer the existence of dysfunction of frontal cortical serotonin 2A receptors from the elevations of whole-blood serotonin levels in groups of people with autism spectrum disorder, [57] a study that assessed serotonin type 2A receptors in the platelets and the brains of the same individuals found no correlation. When using PET after administration of [18 F]setoperone to measure central serotonin type 2A receptors and [3 H]lysergic acid diethylamide to measure serotonin type 2A receptors on the platelets of 12 healthy volunteers, no correlation between binding potentials was found in any one individual. [58]
These recent results challenge the assumption that peripheral platelet serotonin type 2A receptors can be used to represent those in the brains of living human beings.
PET scan studies of the serotonergic system in autism spectrum disorder
Use of PET scanning after administration of [11C]AMT facilitates identification of alterations in metabolism of serotonin in the brains of persons with autism spectrum disorder and related conditions. [45, 68, 69, 70]
Gross abnormalities have been detected by visual inspection of PET scans of boys with autism spectrum disorder after administration of [11C]AMT. Most boys with autism spectrum disorder display decreased accumulation of [11C]AMT in the left frontal cortex and left thalamus and elevated accumulation of [11C]AMT in the right dentate nucleus of the cerebellum. A minority of boys with autism spectrum disorder display a pattern of accumulation of [11C]AMT that is the mirror image of the pattern seen in the majority of boys with autism spectrum disorder (ie, they have decreased accumulation of [11C]AMT in the right frontal cortex and the right thalamus and elevated accumulation of [11C]AMT in the left dentate nucleus of the cerebellum). In addition, the brother of a boy with autism spectrum disorder was a calendar calculator who lined up his toys ritualistically and demonstrated increased accumulation of [11C]AMT in the right dentate nucleus of the cerebellum. [45, 68]
Because deficits of the frontal lobes often are manifested by problems in executive functioning, these findings suggest a physiologic basis for the problems of interpreting and initiating social interactions. In addition, anomalous thalamic findings indicate a functional source for the problems with analyzing environmental stimuli and performing appropriate responses. The anomalies in the dentate nucleus may provide the explanation for the clumsiness and other coordination problems of some boys with autism spectrum disorder. [68]
Furthermore, the findings suggest a deficiency in the pathway connecting the affected frontal lobe with the ipsilateral thalamus and the contralateral cerebellum. Thus, altered serotonergic metabolism in the dentatothalamocortical pathways may be a pathophysiologic mechanism of autism spectrum disorder in some boys. [68]
Seven boys and one girl with autism spectrum disorder and five of their nonautistic siblings were studied after administration of AMT. Asymmetries were observed in all boys with autism spectrum disorder but not in the girl with autism spectrum disorder. Five boys with autism spectrum disorder demonstrated decreased serotonin synthesis in the left frontal cortex and the left thalamus and increased serotonin synthesis in the dentate nucleus of the right cerebellum. The other two boys with autism spectrum disorder demonstrated decreased serotonin synthesis in the right frontal cortex and the right thalamus and increased serotonin synthesis in the dentate nucleus of the left cerebellum. [45, 68]
In contrast with their five nonautistic siblings, the seven boys with autism demonstrated statistically significant asymmetry indices for the frontal cortex, the thalamus, and the dentate nucleus combined and separately for the frontal cortex and the thalamus. [45, 68]
A subsequent study of PET scanning after administration of [11C]AMT to nine girls with autism spectrum disorder revealed asymmetry of frontal cortical synthesis in the four girls without intellectual disability but not in the five girls with intellectual disability. Heterogeneous cerebellar anomalies were observed in three of the four girls with frontal asymmetries. These studies suggest that girls with autism without intellectual disability may exhibit dysregulation of serotonin metabolism similar to boys with autism, whereas girls with autism with intellectual disability may have different frontal serotonin metabolism. Thus, PET studies of serotonin synthesis in autism suggest that serotonin metabolism in autism spectrum disorder varies biologically according to sex and intelligence. [45, 69]
A subsequent study using [11C]AMT confirmed the earlier findings of atypical serotonin synthesis in children, adolescents, and adults with autism. Thirty children with autism, eight of their siblings, and 16 children with epilepsy were evaluated after administration of [11C]AMT. This sample included the eight children with autism and their five siblings described in the previous paragraph. Children aged 2-5 years with typical development exhibited higher serotonin synthesis than that exhibited by adults; as the children grew, the serotonin synthesis gradually declined to adult values. This decline to adult values occurred earlier in girls than in boys. In contrast, boys, but not girls, with autism exhibited decreased focal serotonin synthesis in the frontal cortex and thalamus at all ages. [45]
Chandana and colleagues used [11C]AMT to study 117 children with autism spectrum disorder and found that children with left cortical [11C]AMT decreases showed severe language impairments, and children with right cortical [11C]AMT decreases showed more left and mixed handedness. [50]
In a study that used a radioligand for the serotonin transporter [11C]-labeled trans-1,2,3,5,6,10-beta-hexahydro-6-[4-(methylthio)phenyl]pyrrolo-[2,1-a]isoquinoline ([11C](+)McNeil5652) in 20 men with autism spectrum disorder and 20 age-matched and intelligence quotient (IQ)-matched controls, the uptake throughout the brain was significantly lower in the group with autism spectrum disorder. [59] In addition, impaired social cognition was correlated with reductions of the serotonin transporter in the anterior and posterior cingulate cortices of the men with autism spectrum disorder. These findings suggest the presence of dysfunction of the serotonergic system in a subset of people with autism spectrum disorder. [59] The study requires replication with different larger samples in various geographic locations.
Whole-brain serotonin synthesis is decreased in both boys and girls with autism spectrum disorder as compared to children with typical development, and it gradually increases with age to values 1.5 times greater than those of adults with typical development. In adults with typical development, serotonin synthesis is higher in women than in men. However, inaccuracies are likely because of the failure to employ partial volume corrections to compensate for ventricular enlargement and atrophy or inadequate development reported in the corpus callosum, cerebellum, temporal lobes, and parietal lobes of some individuals with autistic disorder. The partial volume correction is likely to be important because of the unreliability of manually outlining portions of magnetic resonance images (MRIs).
Goldberg and colleagues used [18F]setoperone, a radio-ligand for the serotonin receptor to study 19 parents of children with autism spectrum disorder and 17 adult controls and found decreased cortical serotonergic binding in parents of children with autism spectrum disorder. [71]
Another study with [18F]setoperone in 8 high-functioning adults with autism spectrum disorder (5 men, 3 women) and 12 adult healthy controls (8 men, 4 women) demonstrated decreased thalamic serotonergic receptor binding in adults with autism spectrum disorder and a negative relationship between thalamic serotonergic receptor binding and a history of language impairment in adults with autism spectrum disorder. [72]
In people with tuberous sclerosis and autistic disorder, PET scans with [11C]AMT demonstrate increased uptake bilaterally in the caudate nuclei. [39, 40, 41, 45]
Neurotransmission in autism spectrum disorder will be better understood through investigations with radiotracers for serotonin receptor subtypes including 5-HT1A, 5-HT1B, 5-HT4, and 5-HT6. [1]
Treatment studies with serotonergic agents
Dysfunction of central serotonin receptors and transmitters in some individuals with autism spectrum disorder, possibly through interactions with central glutamate pathways, is suggested by reports of beneficial effects of medications that increase central serotonin, such as 5-HT2A receptor antagonists. However, treatment with serotonergic agents is not consistently beneficial for serious symptoms of autism spectrum disorder, such as self-injury. The symptom provocation strategy of acute depletion of brain serotonin induced by a tryptophan-poor amino-acid load worsened symptoms in a group of adults with autism spectrum disorder, possibly by reducing serotonin levels in the brain.
Hypersensitivity of serotonin type 1D receptor in autism is suggested by the greater growth hormone response and increase of repetitive behavior of a small sample of adults with autism spectrum disorder treated with sumatriptan, a serotonin type 1D agonist, as compared to placebo. [60] Furthermore, development of serotonin transporter regulatory agents may result in new therapies targeted at specific deficits of serotonin metabolism of a group of people with autism spectrum disorder.
Chugani and colleagues studied 166 participants (aged 2 to < 6 years) with autism spectrum disorder using [11C]AMT and found better response to 2.5-mg buspirone twice daily in children with fewer foci of increased brain tryptophan. Buspirone, a 5HT1A serotonin partial agonist was used in this clinical trial. Since serotonin plays an important role in post-natal brain development, the use of serotonin agonists in children younger than 6 years of age could help in the treatment of key features of autism spectrum disorder. Therefore, this study provides a background for further exploration of this treatment as a therapy to target repetitive and restrictive behavior along with early behavioral intervention. [70]
Oxytocin is a neuropeptide whose mechanism of action remains unclear. Serotonin and oxytocin systems are functionally and anatomically connected. [74, 75] Together they control emotion-based behaviors. In a clinical trial, a radio-ligand [11C]-3-amino-4-(2-[(dimethylamino)methyl]phenylthio)benzonitrile ([11C]DASB) was used to study ten adult men with autism spectrum disorder aged 23 to 41 years. Increased serotonin transporter binding was found in the left inferior frontal gyrus extending to the left middle frontal gyrus of men with autism spectrum disorder after administration of oxytocin intra-nasally for eight to ten weeks. [73]
Another clinical trial also used [11C]DASB, a radio-ligand for serotonin transporter in ten adult men aged 23 to 41 years with autism spectrum disorder and found increased serotonin transporter level in the striatum that correlated with increased negative emotional response to human faces after treatment with 12 IU of intranasal oxytocin twice daily for 10 weeks. Increased serotonin level in the right striatum correlated with increased hostility after 12 IU of intranasal oxytocin twice daily for 10 weeks. [76]
PET Scanning of the Dopaminergic System
Another potential use of positron emission tomography (PET) scanning in autism spectrum disorder is evaluation of the dopaminergic system. In a study that used a radioligand for the dopamine transporter, (-)-2-beta-carbomethoxy-3-beta-(4-fluorophenyl) tropane (CFT), also known as [11C]WIN-35,428, in 20 men with autism spectrum disorder aged 18 to 26 years and age-matched and intelligence quotient (IQ)-matched controls, the uptake in the orbitofrontal cortex was significantly higher in the group with autism spectrum disorder. [59] The study requires replication with different larger samples in various geographic locations.
Ernst and colleagues studied 14 medication-free children with autism spectrum disorder aged 13 years (8 boys, 6 girls) and 10 healthy children aged 14 years (7 boys, 3 girls) using radio-ligand [18F]fluorodopa and found decreased dopaminergic transmission in anterior medial prefrontal cortex of children with autism spectrum disorder. [77]
Xiao-Mian and colleagues studied children with autism spectrum disorder (10 boys) aged 3 to 10 years and 10 age-matched and gender-matched controls using technetium, 2-[[2-[[[[3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3,2,1 ]oct-2-yl]-methyl](2-mercaptoethyl)amino]ethyl]amino]ethanethiolato(3-)]-oxo-[1R-(exo-exo)] also known as [99mTc]TRODAT-1. They found increased dopamine transporter binding in whole brain of children with autism spectrum disorder. [78]
These findings suggest the presence of dysfunction of the dopaminergic system in a subset of people with autism. PET scanning also plays an important role in clinical trials involving dopaminergic system.
Tetrahydrobiopterin is a cofactor of the three aromatic amino acid hydroxylase enzymes [80] , used in the degradation of phenylalanine and in the biosynthesis of the neurotransmitters serotonin, dopamine, norepinephrine, epinephrine, melatonin, and is a cofactor for the production of nitric oxide (NO) by the nitric oxide synthases. [81] Fernell and colleagues studied 6 children with infantile autism aged 3 to 5 years using radio-ligands H215O, L-[11C]DOPA, and (3-N-[11C]methyl)spiperone ([11C]NMSP). Tetrahydrobiopterin was administered in sachet twice daily in the dosage of 3mg/kg/day for 3 months. Treatment with tetrahydrobiopterin resulted in 10% decreased dopamine D2 receptor binding in the striatum. [79]
Research is needed to investigate the effects of antipsychotic and psychostimulant medications on neurotransmission. Future investigations are needed to identify if the dopamine transporter is blocked by methylphenidate, a medication commonly administered to people with autism spectrum disorder to treat symptoms of attention-deficit/hyperactivity disorder (ADHD). Additionally, neurotransmission in autism spectrum disorder will by elucidated by the use of radiotracers for dopamine D1, D2, and D3 receptors and the high-affinity state of dopamine D2 and D3 receptors. [1]
PET Scanning of the Glutamatergic System
Fatemi and colleagues studied six men with autism and three healthy controls using a radio-ligand for glutamate receptor, [18F]-3-fluoro-5-[(pyridin-3-yl)ethynyl]benzonitrile, also known as [18F]FPEB. PET scanning demonstrated increased metabotropic glutamate receptor subtype 5 in postcentral gyrus and cerebellum of men with autism. [82]
PET Scanning of the GABAergic System
Dysfunction of gamma-amino-butyric acid (GABA), the most abundant inhibitory neurotransmitter in the human brain, likely plays a role in the pathophysiology of autism spectrum disorder. PET scanning demonstrated reductions in the α5 subtype of the GABA type A receptors bilaterally in the amygdala and the nucleus accumbens, two limbic regions, of three men with high-functioning autism spectrum disorder in contrast to three age-matched men with typical development. [61] Because these regions play a role in the reward system, this dysfunction may be related to the social and behavioral anomalies of men with autism spectrum disorder. [8]
Another study used radio-ligands, [11C]flumazenil in 15 adults with autism spectrum disorder and in 15 controls, and [11C]C15H14N6O3 also known as [11C]Ro15-4513 in 12 adults with autism spectrum disorder and in 16 controls. The study showed no differences in GABAA receptor or GABAA α5 subunit availability in any brain region of adults with or without autism spectrum disorder. [83]
PET Scanning and Rett Syndrome
Rett syndrome is a pervasive developmental disorder presenting in early childhood primarily in girls. Children with Rett syndrome exhibit normal development in the first year of life. They typically fail to attain milestones between ages 1 and 6 years. They exhibit profound intellectual disability and typically require assistance in the activities of daily living. Almost all individuals with Rett syndrome display abnormalities of the methyl-CpG-binding protein 2 (MeCP2) gene. [26] Women with Rett syndrome demonstrate less acetylcholine in the brain than healthy adults. [27] Reductions in acetylcholine are correlated with reduced abilities to perform activities of daily living in women with Rett syndrome. [27]
See the image below.
 Visual expressions of mean uptake on single-photon emission-computed tomography (SPECT) 24 h following the intravenous administration of approximately 333 MBq (9 mCi) (2)-5-[123I]iodobenzovesamicol ([123I]IBVM), a radioligand for vesicular acetylcholine transporters to eight healthy adults (upper row) and four women with Rett syndrome (lower row). The left of each panel corresponds to the left of the brain. The panels represent transverse sections of the brain at the level of the cerebellum (left), striatum (center), and cingulated gyrus (right). The lower row illustrates the reduced uptake in the women with Rett syndrome in the vermis and the bilateral precentral cortices (lower left panel), the striatum (lower central panel), and the middle cingulate gyrus (right lower panel). Used with permission from Brasic JR, et al. Correlation of the vesicular acetylcholine transporter densities in the striata to the clinical abilities of women with Rett syndrome. Synapse. Jun 2012;66(6):471-82.
Visual expressions of mean uptake on single-photon emission-computed tomography (SPECT) 24 h following the intravenous administration of approximately 333 MBq (9 mCi) (2)-5-[123I]iodobenzovesamicol ([123I]IBVM), a radioligand for vesicular acetylcholine transporters to eight healthy adults (upper row) and four women with Rett syndrome (lower row). The left of each panel corresponds to the left of the brain. The panels represent transverse sections of the brain at the level of the cerebellum (left), striatum (center), and cingulated gyrus (right). The lower row illustrates the reduced uptake in the women with Rett syndrome in the vermis and the bilateral precentral cortices (lower left panel), the striatum (lower central panel), and the middle cingulate gyrus (right lower panel). Used with permission from Brasic JR, et al. Correlation of the vesicular acetylcholine transporter densities in the striata to the clinical abilities of women with Rett syndrome. Synapse. Jun 2012;66(6):471-82.
The dopamine transporter ligand, (-)-2-beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (CFT), also known as [11 C]WIN-35,428, has been used to study patients with Rett disorder and healthy control subjects. Patients with Rett disorder demonstrate reduction in the binding potential of CFT in the caudate and the putamen.
Low normal values of receptor density of postsynaptic dopamine D2 receptors in the caudate of participants with Rett syndrome after the administration of 3-N -[11C]methylspiperone (NMS) [62] was substantiated by our finding reduced dopamine D2 receptors in the striatum of participants with Rett syndrome. [93] Nevertheless, other researchers using single-photon emission computed tomography (SPECT) scanning after administration of [123 I]iodolisuride found increased specific binding of this radiotracer to the dopamine D2 receptor in participants with Rett syndrome.These results suggest dysfunction in dopamine transport in the caudate and putamen of people with Rett syndrome and provide a basis for explaining the abnormal movements and postures observed in this condition
The discrepancies among the groups suggest that Rett syndrome has heterogeneous causes and manifestations. Increased and decreased dopamine-receptor binding may characterize different classes of people with Rett syndrome. Alternatively, dopamine receptor binding may change at different stages in the progression of Rett syndrome. Another explanation is that different findings may result from the specific protocols, including radiotracers, used by the research groups.
Other PET Scanning Clinical Studies
The regional cerebral blood flow (rCBF) is estimated by radioisotope brain imaging. Hexamethylpropyleneamine oxide (HMPAO), a lipophilic agent, typically is labeled with technetium-99m [99m Tc]. Deficits have been demonstrated in individuals with autism spectrum disorder; however, no anomaly has been found consistently. Some people with autism spectrum disorder have exhibited reduced rCBF in the vermis, cerebellar hemisphere, thalami, basal ganglia, and parietal and temporal lobes. These abnormalities may be clarified by future studies to identify biological subgroups.
In a study that assessed rCBF, dopamine synthesis rate, and dopamine D2 receptor binding in 6 children aged 3-5 years utilizing oxygen-15 [15O] water (12 MBq/kg), carbon-11–labeled dihydroxyphenylalanine (L-[11C]DOPA) (6 MBq/kg), and 3-N -[11C] methylspiperone ([11C]NMS) [62] (4 MBq/kg), respectively, the rCBF did not differ from that of adults. The children were treated with 6R-L-erythro-5,6,7,8-tetrahydrobiopterin (RBH4), a cofactor for tyrosine hydroxylase in the biosynthetic pathway of catecholamines and dopamine, at a dose of 3 mg/kg/day with twice-daily oral administrations for 12 weeks in an open fashion.
The [11C]DOPA utilization rate and [11C]NMS binding were elevated in all participants before treatment with RBH4. After treatment, [11C]NMS binding in the caudate was reduced by 10%, and that in the putamen was reduced by 8%. The L-[11C]DOPA utilization rate remained unchanged. The increased dopamine D2 receptor binding seen before treatment was reduced by the therapy.
Oxygen-15 water
Positron emission tomography (PET) scans after intravenous (IV) administration of 25 mCi of [15O] water as a slow bolus over 20 seconds revealed reversal of hemispheric dominance during verbal auditory stimulation, reduced auditory dominance activation during verbal auditory stimulation, and reduced cerebellar activation during nonverbal auditory perception in adults with high-functioning autism spectrum disorder, suggesting cerebellar dysfunction and atypical dominance for language.
PET scans after IV bolus administration of 0.5 or 7 mCi of [15O] water revealed hypoperfusion in the left, right, or both temporal lobes in children with autism spectrum disorder.
PET scans after IV bolus administration of 300 MBq of [15O] water revealed increasing rCBF: (1) in the medial prefrontal cortex with increasing horizontal gaze aversion and (2) in the superior and medial temporal gyri with increasing direct gaze in a group of healthy women. These findings are relevant to the understanding of autism spectrum disorder, because gaze is hypothesized to reflect the ability to detect the mental states of others. People with high-functioning autism spectrum disorder, Asperger syndrome, and related disorders frequently demonstrate impaired ability to comprehend the mental states of others. The ability to understand the mental states of others has been termed the ability to formulate a theory of mind.
The deficits in formulating a theory of mind for others have been further investigated by PET scans after IV bolus administration of [15O] water to adults with high-functioning autism spectrum disorder or Asperger syndrome and to healthy volunteers while watching animated sequences. While both groups identified random and goal-directed movements, the subjects with high-functioning autism spectrum disorder or Asperger syndrome displayed impaired ability to interpret interactions among moving figures with implied intentions. PET scans during the animations with implied intentions demonstrated more activation in the medial prefrontal cortex, the superior temporal sulcus at the temporoparietal junction, and the temporal poles in the healthy adult volunteers than in the subjects with high-functioning autism spectrum disorder or Asperger syndrome.
Although increased activation in the extrastriate region was present in both groups during the animations with implied intentions, people with high-functioning autism spectrum disorder or Asperger syndrome had reduced functional connectivity between the extrastriate region and the superior temporal sulcus at the temporoparietal junction. These results suggest a neuroanatomic hypothesis for the impaired ability of people with high-functioning autism spectrum disorder and Asperger syndrome to recognize the mental states of others.
Multimodal Imaging in Autistic Disorder
Although positron emission tomography (PET) scans are useful to identify regional cerebral metabolism (rCBF) and the distribution of neuroreceptors, they do not provide a comprehensive picture of cerebral functioning in people with autism spectrum disorder. For a thorough evaluation of brain metabolism and physiology, multimodal imaging (ie, assessment of cerebral function through nuclear, radiologic, electrophysiologic, and other techniques simultaneously) is helpful. [84, 86]
PET scanning and video imaging
Filming patients before, during, and after scans provides crucial clinical data about their signs. [87]
PET scanning and radiologic imaging
Radiologic techniques can broadly be divided into structural techniques to identify anatomic structures and functional techniques to identify physiologic activities. Structural radiologic techniques include magnetic resonance imaging (MRI), computed tomography (CT) scanning, and radiography. Functional radiologic techniques include functional MRI (fMRI) and magnetic resonance spectroscopy (MRS). [63, 32, 33]
PET scanning and structural imaging
A practical example of multimodal imaging is the use of a radiologic technique, such as MRI or CT scanning, and PET scanning. MRI and CT scanning are optimal tools to identify the anatomic structures of the brain, whereas PET is a good way to identify the physiology of the brain. The findings may be compared by having the subject wear a face mask with fiducial markers during both the MRI or CT scan and the PET scan. The mask allows subsequent coregistration of the MRI or CT scan and the PET scan and, thus, superimposition of the anatomic and physiologic images. However, coregistration is a time-consuming procedure, and error may be introduced in the attempt to superimpose images that are not identical.
The newly developed PET-CT scanning resolves the problem of coregistration by simultaneously producing both images. The joint use of PET scanning and radiologic techniques is crucial to incorporate the strengths of both functional and structural imaging procedures. Because structural techniques demonstrate increments in the volume of both gray and white matter in people with autism spectrum disorder, [64] multimodal imaging use of both PET scanning and structural techniques is indicated. [63]
PET scanning and functional imaging
Because functional radiological procedures have identified reduced activity in the perigenual anterior cingulate cortex when individuals perform social tasks and in the dorsal anterior cingulate cortex when they perform nonsocial tasks, [65] the use of PET scanning and other functional imaging procedures is a promising tool to understand the pathophysiology of autism spectrum disorder. Functional imaging techniques include nuclear medical procedures such as PET and single photon emission computed tomography (SPECT) scanning, as well as radiologic procedures including fMRI and MRS. [63, 8]
PET scanning and electrophysiologic imaging
Another type of multimodal imaging is the use of psychophysiologic and electrophysiologic measures in conjunction with PET scanning. For example, a neurometric evaluation includes electroencephalography (EEG), visual evoked potentials (VEPS), cortical auditory evoked potentials (CAEPs), auditory cognitive evoked potentials (P300), and somatosensory evoked potentials (SEPs). The neurometric protocol can be performed after PET evaluation to provide a view of the electrical activity of the different portions of the brain along with the physiologic assessment provided by PET scanning.
For example,. a boy with autism spectrum disorder and unspecified intellectual disability illustrated by PET scans (see PET Scanning and Metabolism in Autism) (see the images below) was also evaluated by a neurometric tests.
 This image was obtained approximately 30 minutes after intravenous administration of 4.15 mCi of [18F]fluoro-2-deoxyglucose (FDG) to a 10-year-old boy with autism spectrum disorder and unspecified intellectual disabitliy using auto-attenuation with a 24-minute acquisition period. Coronal sections are shown from anterior to posterior; the left side of the figure corresponds to the right side of the patient. Courtesy of Rashid A Fawwaz, MD, PhD, The Kreitchman PET Center, Columbia-Presbyterian Medical Center, New York, NY.
This image was obtained approximately 30 minutes after intravenous administration of 4.15 mCi of [18F]fluoro-2-deoxyglucose (FDG) to a 10-year-old boy with autism spectrum disorder and unspecified intellectual disabitliy using auto-attenuation with a 24-minute acquisition period. Coronal sections are shown from anterior to posterior; the left side of the figure corresponds to the right side of the patient. Courtesy of Rashid A Fawwaz, MD, PhD, The Kreitchman PET Center, Columbia-Presbyterian Medical Center, New York, NY.
 This image was obtained approximately 30 minutes after intravenous administration of 4.15 mCi of [18F]fluoro-2-deoxyglucose (FDG) to a 10-year-old boy with autism spectrum disorder and unspecified intellectual disability using auto-attenuation with a 24-minute acquisition period. Axial sections are shown from superior to inferior; the left side of the figure corresponds to the right side of the patient. Hypometabolism, greater on the left than the right, is demonstrated in the temporal and parietal regions. This is particularly prominent in the third and fourth images. Courtesy of Rashid A Fawwaz, MD, PhD, The Kreitchman PET Center, Columbia-Presbyterian Medical Center, New York, NY.
This image was obtained approximately 30 minutes after intravenous administration of 4.15 mCi of [18F]fluoro-2-deoxyglucose (FDG) to a 10-year-old boy with autism spectrum disorder and unspecified intellectual disability using auto-attenuation with a 24-minute acquisition period. Axial sections are shown from superior to inferior; the left side of the figure corresponds to the right side of the patient. Hypometabolism, greater on the left than the right, is demonstrated in the temporal and parietal regions. This is particularly prominent in the third and fourth images. Courtesy of Rashid A Fawwaz, MD, PhD, The Kreitchman PET Center, Columbia-Presbyterian Medical Center, New York, NY.
 This image was obtained approximately 30 minutes after intravenous administration of 4.15 mCi of [18F]fluoro-2-deoxyglucose (FDG) to a 10-year-old boy with autism spectrum disorder and unspecified intellectual disability using auto-attenuation with a 24-minute acquisition period. Sagittal images are seen from right to left. Courtesy of Rashid A Fawwaz, MD, PhD, The Kreitchman PET Center, Columbia-Presbyterian Medical Center, New York, NY.
This image was obtained approximately 30 minutes after intravenous administration of 4.15 mCi of [18F]fluoro-2-deoxyglucose (FDG) to a 10-year-old boy with autism spectrum disorder and unspecified intellectual disability using auto-attenuation with a 24-minute acquisition period. Sagittal images are seen from right to left. Courtesy of Rashid A Fawwaz, MD, PhD, The Kreitchman PET Center, Columbia-Presbyterian Medical Center, New York, NY.
When the same boy with autism spectrum disorder was aged 8 years, an electrophysiologic protocol was obtained. The series of images from this boy that are provided below illustrate a slightly abnormal neurometric electrophysiologic evaluation that is similar to some children with learning disabilities. Neurometric EEG evaluation is not currently clinically indicated in patients with autism spectrum disorder and requires further data to clarify its clinical utility. These images were provided through the courtesy of E. Roy John, PhD, Neurometrician, Brain Research Laboratories, Department of Psychiatry, Bellevue Hospital Center and the New York University School of Medicine, New York, NY.
The following image illustrates a page of the EEG, which demonstrates predominant theta activity in the patient. Alpha activity predominates in the posterior regions and the vertex. No paroxysmal events are noted. Shortly before age 14 years, he developed nocturnal seizures.
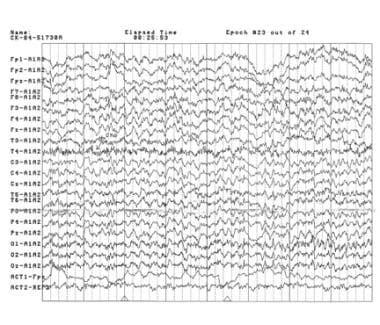 A page of an electroencephalogram of an 8-year-old boy with autism spectrum disorder and unspecified intellectual disability obtained in the awake resting state with the eyes closed. Theta activity predominates in this tracing. Alpha activity is predominant in the posterior regions and the vertex. No paroxysmal events are noted. Electromyographic artefacts are present in the ninth and tenth lines. Courtesy of E Roy John, PhD, Neurometrician, Brain Research Laboratories, Department of Psychiatry, Bellevue Hospital Center and the New York University School of Medicine, New York, NY.
A page of an electroencephalogram of an 8-year-old boy with autism spectrum disorder and unspecified intellectual disability obtained in the awake resting state with the eyes closed. Theta activity predominates in this tracing. Alpha activity is predominant in the posterior regions and the vertex. No paroxysmal events are noted. Electromyographic artefacts are present in the ninth and tenth lines. Courtesy of E Roy John, PhD, Neurometrician, Brain Research Laboratories, Department of Psychiatry, Bellevue Hospital Center and the New York University School of Medicine, New York, NY.
The image below illustrates the neurometric quantitative EEG of this child with autism spectrum disorder. The first row demonstrates diffuse low absolute power in all leads in all bands, reaching significance for delta activity on the left side at central, parietal, and posterior temporal regions. The second row demonstrates the relative power. Nonsignificant diffuse excess of theta activity can be observed on the anterior regions of the left hemisphere (second row). Deficits of theta and delta and an excess of alpha at the vertex, also in the second row, are significant. The interhemispheric power asymmetry illustrated in the third row is normal. The fourth row illustrates an extremely abnormal bipolar frontal-temporal hypocoherence in beta activity. The interhemispheric coherence is otherwise normal (fourth row).
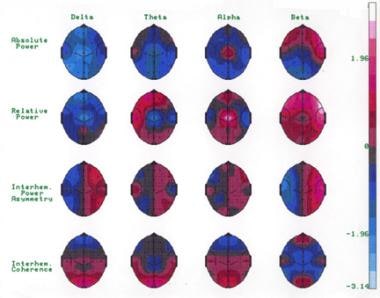 The quantitative electroencephalogram of an 8-year-old boy with autism autism spectrum disorder and unspecified intellectual disability obtained in the awake resting state with the eyes closed. Diffuse low absolute power in all leads in all bands is observed (first row). The absolute power reaches significance at the P < 0.05 level for delta activity on the left side at central, parietal, and posterior temporal regions (first row). In relative power, a diffuse excess of theta activity occurs on the anterior regions of the left hemisphere, not reaching statistical significance (second row). Furthermore, a significant deficit of theta and delta activity and a significant excess of alpha activity at the vertex also can be observed (P < 0.01) in the second row. The generalized increase of beta activity reaching significance at F7, F8, and T4 probably represents muscle tension (second row). The interhemispheric power asymmetry in the third row is normal. The fourth row demonstrates extremely abnormal hypocoherence in beta activity in the bipolar frontal-temporal regions (fourth row). The interhemispheric coherence is otherwise normal (fourth row). Courtesy of E Roy John, PhD, Neurometrician, Brain Research Laboratories, Department of Psychiatry, Bellevue Hospital Center and the New York University School of Medicine, New York, NY.
The quantitative electroencephalogram of an 8-year-old boy with autism autism spectrum disorder and unspecified intellectual disability obtained in the awake resting state with the eyes closed. Diffuse low absolute power in all leads in all bands is observed (first row). The absolute power reaches significance at the P < 0.05 level for delta activity on the left side at central, parietal, and posterior temporal regions (first row). In relative power, a diffuse excess of theta activity occurs on the anterior regions of the left hemisphere, not reaching statistical significance (second row). Furthermore, a significant deficit of theta and delta activity and a significant excess of alpha activity at the vertex also can be observed (P < 0.01) in the second row. The generalized increase of beta activity reaching significance at F7, F8, and T4 probably represents muscle tension (second row). The interhemispheric power asymmetry in the third row is normal. The fourth row demonstrates extremely abnormal hypocoherence in beta activity in the bipolar frontal-temporal regions (fourth row). The interhemispheric coherence is otherwise normal (fourth row). Courtesy of E Roy John, PhD, Neurometrician, Brain Research Laboratories, Department of Psychiatry, Bellevue Hospital Center and the New York University School of Medicine, New York, NY.
The following image illustrates a normal left brainstem auditory evoked potential for this boy.
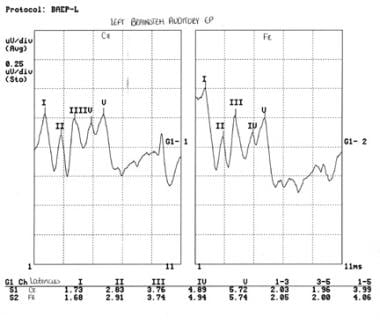 Left brainstem auditory evoked potential of an 8-year-old boy with autism spectrum disorder and unspecified intellectual disability. The tracing is normal for peak and interpeak latencies and morphology. Courtesy of E Roy John, PhD, Neurometrician, Brain Research Laboratories, Department of Psychiatry, Bellevue Hospital Center and the New York University School of Medicine, New York, NY.
Left brainstem auditory evoked potential of an 8-year-old boy with autism spectrum disorder and unspecified intellectual disability. The tracing is normal for peak and interpeak latencies and morphology. Courtesy of E Roy John, PhD, Neurometrician, Brain Research Laboratories, Department of Psychiatry, Bellevue Hospital Center and the New York University School of Medicine, New York, NY.
The image below illustrates the same child's normal auditory cognitive evoked potentials recorded in response to common and rare tones (P300).
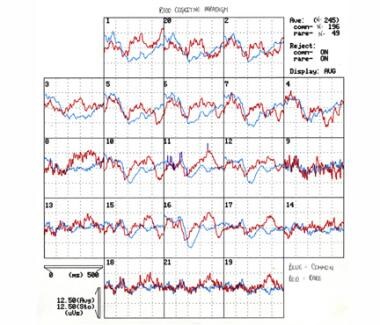 Auditory cognitive evoked potentials recorded in response to common and rare tones (P300) of an 8-year-old boy with autism spectrum disorder and unspecified intellectual disability. The differences between evoked potentials to common and rare stimuli (P300) are normal. Courtesy of E Roy John, PhD, Neurometrician, Brain Research Laboratories, Department of Psychiatry, Bellevue Hospital Center and the New York University School of Medicine, New York, NY.
Auditory cognitive evoked potentials recorded in response to common and rare tones (P300) of an 8-year-old boy with autism spectrum disorder and unspecified intellectual disability. The differences between evoked potentials to common and rare stimuli (P300) are normal. Courtesy of E Roy John, PhD, Neurometrician, Brain Research Laboratories, Department of Psychiatry, Bellevue Hospital Center and the New York University School of Medicine, New York, NY.
Additionally instrumentation to provide quantitative continuous measurements of movements [85] of people with autism spectrum disorders will provide crucial data to facilitate diagnostic and therapeutic interventions before, during, and after scans and other interventions.
-
This image was obtained approximately 30 minutes after intravenous administration of 4.15 mCi of [18F]fluoro-2-deoxyglucose (FDG) to a 10-year-old boy with autism spectrum disorder and unspecified intellectual disabitliy using auto-attenuation with a 24-minute acquisition period. Coronal sections are shown from anterior to posterior; the left side of the figure corresponds to the right side of the patient. Courtesy of Rashid A Fawwaz, MD, PhD, The Kreitchman PET Center, Columbia-Presbyterian Medical Center, New York, NY.
-
This image was obtained approximately 30 minutes after intravenous administration of 4.15 mCi of [18F]fluoro-2-deoxyglucose (FDG) to a 10-year-old boy with autism spectrum disorder and unspecified intellectual disability using auto-attenuation with a 24-minute acquisition period. Axial sections are shown from superior to inferior; the left side of the figure corresponds to the right side of the patient. Hypometabolism, greater on the left than the right, is demonstrated in the temporal and parietal regions. This is particularly prominent in the third and fourth images. Courtesy of Rashid A Fawwaz, MD, PhD, The Kreitchman PET Center, Columbia-Presbyterian Medical Center, New York, NY.
-
This image was obtained approximately 30 minutes after intravenous administration of 4.15 mCi of [18F]fluoro-2-deoxyglucose (FDG) to a 10-year-old boy with autism spectrum disorder and unspecified intellectual disability using auto-attenuation with a 24-minute acquisition period. Sagittal images are seen from right to left. Courtesy of Rashid A Fawwaz, MD, PhD, The Kreitchman PET Center, Columbia-Presbyterian Medical Center, New York, NY.
-
A page of an electroencephalogram of an 8-year-old boy with autism spectrum disorder and unspecified intellectual disability obtained in the awake resting state with the eyes closed. Theta activity predominates in this tracing. Alpha activity is predominant in the posterior regions and the vertex. No paroxysmal events are noted. Electromyographic artefacts are present in the ninth and tenth lines. Courtesy of E Roy John, PhD, Neurometrician, Brain Research Laboratories, Department of Psychiatry, Bellevue Hospital Center and the New York University School of Medicine, New York, NY.
-
The quantitative electroencephalogram of an 8-year-old boy with autism autism spectrum disorder and unspecified intellectual disability obtained in the awake resting state with the eyes closed. Diffuse low absolute power in all leads in all bands is observed (first row). The absolute power reaches significance at the P < 0.05 level for delta activity on the left side at central, parietal, and posterior temporal regions (first row). In relative power, a diffuse excess of theta activity occurs on the anterior regions of the left hemisphere, not reaching statistical significance (second row). Furthermore, a significant deficit of theta and delta activity and a significant excess of alpha activity at the vertex also can be observed (P < 0.01) in the second row. The generalized increase of beta activity reaching significance at F7, F8, and T4 probably represents muscle tension (second row). The interhemispheric power asymmetry in the third row is normal. The fourth row demonstrates extremely abnormal hypocoherence in beta activity in the bipolar frontal-temporal regions (fourth row). The interhemispheric coherence is otherwise normal (fourth row). Courtesy of E Roy John, PhD, Neurometrician, Brain Research Laboratories, Department of Psychiatry, Bellevue Hospital Center and the New York University School of Medicine, New York, NY.
-
Left brainstem auditory evoked potential of an 8-year-old boy with autism spectrum disorder and unspecified intellectual disability. The tracing is normal for peak and interpeak latencies and morphology. Courtesy of E Roy John, PhD, Neurometrician, Brain Research Laboratories, Department of Psychiatry, Bellevue Hospital Center and the New York University School of Medicine, New York, NY.
-
Auditory cognitive evoked potentials recorded in response to common and rare tones (P300) of an 8-year-old boy with autism spectrum disorder and unspecified intellectual disability. The differences between evoked potentials to common and rare stimuli (P300) are normal. Courtesy of E Roy John, PhD, Neurometrician, Brain Research Laboratories, Department of Psychiatry, Bellevue Hospital Center and the New York University School of Medicine, New York, NY.
-
Visual expressions of mean uptake on single-photon emission-computed tomography (SPECT) 24 h following the intravenous administration of approximately 333 MBq (9 mCi) (2)-5-[123I]iodobenzovesamicol ([123I]IBVM), a radioligand for vesicular acetylcholine transporters to eight healthy adults (upper row) and four women with Rett syndrome (lower row). The left of each panel corresponds to the left of the brain. The panels represent transverse sections of the brain at the level of the cerebellum (left), striatum (center), and cingulated gyrus (right). The lower row illustrates the reduced uptake in the women with Rett syndrome in the vermis and the bilateral precentral cortices (lower left panel), the striatum (lower central panel), and the middle cingulate gyrus (right lower panel). Used with permission from Brasic JR, et al. Correlation of the vesicular acetylcholine transporter densities in the striata to the clinical abilities of women with Rett syndrome. Synapse. Jun 2012;66(6):471-82.
-
This figure shows a woman positioned to enter the chamber of a high-resolution tomography (HRRT). (Alveena B. Syed photographed by James Robert Brašić)
Tables
What would you like to print?
- Overview
- Evaluation of Autism Spectrum Disorder
- Screening for PET Scanning
- PET Scanning Overview
- PET Scanning and Metabolism in Autism Spectrum Disorder
- PET Scanning of the Serotonergic System
- PET Scanning of the Dopaminergic System
- PET Scanning of the Glutamatergic System
- PET Scanning of the GABAergic System
- PET Scanning and Rett Syndrome
- Other PET Scanning Clinical Studies
- Multimodal Imaging in Autistic Disorder
- Show All
- Media Gallery
- References










